Redox Reaction: Redox reaction is actually means any reaction which involved electrons transfers.


















The Expertiment : The Oxidation of Alchohols.
Apparatus and chemicals :
Eye protection
Measuring cylinder (5 or 10 cm3)
Well-plate (24 wells) – eg Sigma ref: M9655.
Glass rod
Plastic dropping pipettes, 6

The Oxidation of alchohol.
a Take approximately 2 cm3 of potassium dichromate(VI) solution in a measuring cylinder and add about 1 cm3 of dilute sulfuric acid. Stir with a glass rod.
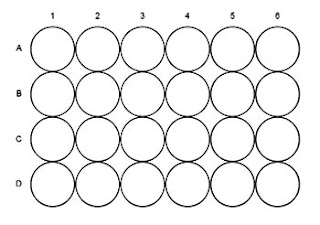
b Put 10 drops of the acidified potassium dichromate(VI) solution into each of the wells
A1 – A4 and B2 (see diagram below).
c Practice (over a sink) producing single drops of ethanol from the pipette.
d Add two drops of the alcohols to the wells as follows:
Well No. | Alcohol |
A1 | Ethanol |
A2 | Propan-1-ol |
A3 | Propan-2-ol |
A4 | 2-Methylpropan-2-ol |
The following alcohols in dropping pipettes:
Ethanol (Highly flammable) or Industrial denatured alcohol, IDA (Highly flammable, Harmful)
Propan-1-ol (Irritant, Highly flammable)
Propan-2-ol (Irritant, Highly flammable)
2-Methylpropan-2-ol (Harmful, Highly flammable), or other tertiary alcohol
Potassium dichromate(VI) solution, 0.1 mol dm–3 (Toxic at this concentration), 2 cm3
Sulfuric acid, 1 mol dm–3 (Irritant at this concentration), 1 cm3


No comments:
Post a Comment